Aluminum electrolytic capacitors are the most common and polarized capacitors. Let’s discuss what affects the leakage current of aluminum electrolytic capacitors.
1.1 Leakage current and its characterization parameters
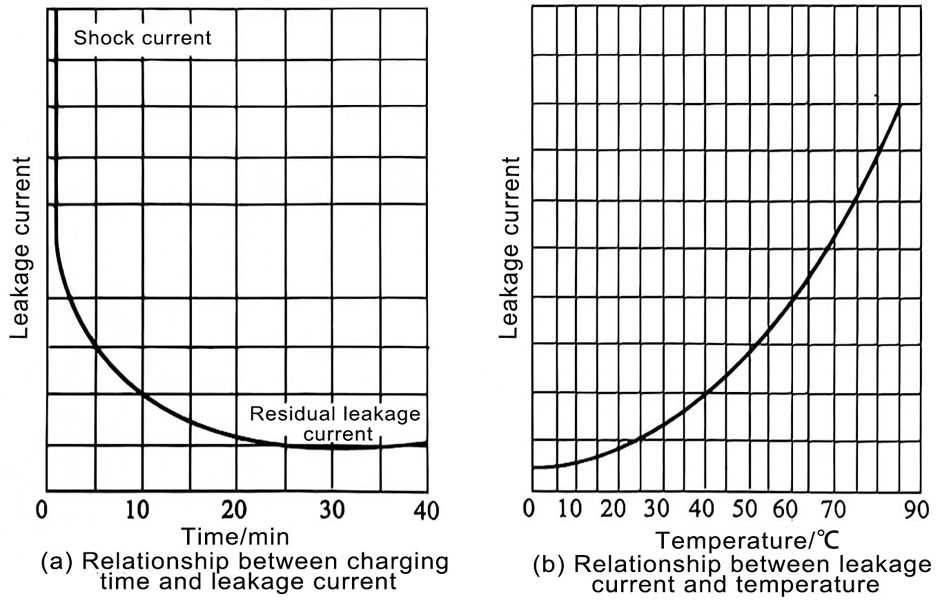
When a constant DC voltage is applied across the capacitor, its charging current starts to be very large and gradually decreases over time. However, the decline will not continue indefinitely, but will reach a more stable state after reaching a certain final value. This final value is called the leakage current, or simply the leakage current IL. During testing, the leakage current of electrolytic capacitors is generally related to the following factors:
(1) The level of applied DC voltage;
(2) The capacitance of the capacitor itself;
(3) The length of time the voltage is applied (applying voltage for a longer time can improve the quality of the oxide film);
(4)The environment in which the capacitor is located;
(5) The type of base metal of the capacitor and the product manufacturing process.
In order to facilitate the comparison of the leakage current of various types of electrolytic capacitors under different voltages and capacitances, the leakage current constant is defined
as follows:
K=I/CU (7-77)
The leakage current constant unit is generally uA/(V·uF). The value of K can be derived from the parallel equivalent circuit
K=U/R·1/CU=1/RL (7-78)
Among them, R is the leakage resistance of the capacitor. In resistor-capacitor parallel circuits, RC is generally called the time constant. Therefore, from equation (7-78), we can know that the leakage current constant is the reciprocal of the capacitor time constant. For aluminum electrolytic capacitors, it can be deduced based on the properties of its oxide layer
R=Pd/A (7-79)
In the formula: P is the resistivity of the oxide layer; d is the thickness of the oxide layer; A is the effective surface area of the oxide film. and
C=εoεrA/d (7-80)
but
K=1/εoεrPv (7-81)
Generally speaking, for the aluminum oxide layer, its resistivity is about 10^13~10^14Ω·cm, and its dielectric constant is about 10. Substituting it can be obtained
K≈0.1~0.01[uA(V·uF)] (7 -82)
The upper limit generally corresponds to the K value specified for ordinary products, and the lower limit is approximate to the K value of long-life products.
From the above analysis, it can be seen that the leakage current constant of aluminum electrolytic capacitors is closely related to the insulation quality of its anodized film. The oxide film medium of the actual device is not a perfect ultra-thin film, and there are more or less various tiny flaws, holes, gaps and other defects on its surface. The leakage current of the product consists of impurity ion current and electron current passing through these defects. For liquid electrolyte type capacitors, since the oxide film is always in the working electrolyte, the contact between the electrolyte and the oxide film is very good and can be repaired at any time, so the leakage current constant is extremely small, as low as 2X10^-3uA/(uF· V). For solid electrolyte type capacitors, there is more or less poor contact between the oxide film and the solid electrolyte. In addition, the solid electrolyte has almost no ability to repair the oxide film, so the leakage current constant is large, up to more than 0.2uA/(uF· V).
1.2 Effect of material purity on leakage current
Material purity has a strong impact on leakage current. Impurities will make the generated oxide film susceptible to electrolyte erosion, so the purity of raw materials should be improved as much as possible during the manufacturing process. When the purity of the anode foil is increased from 99.9% to over 99.99%, the leakage current of the manufactured capacitor can be reduced by an order of magnitude. In addition, chloride ions have a great destructive effect on the oxide film, so the purity of the electrolyte also has a great impact on the leakage current. On the basis of using 99.999% high-purity aluminum foil, ultrapure water with a resistivity of 18.2 Ma·cm, extremely low chloride ion content in chemicals, and strict requirements for purification in the process operation room, extremely low leakage current parameters can be obtained. The leakage current of aluminum electrolytic capacitors at room temperature can be as low as K-0.001uA/(uF·V) or even K=0.0001uA/(uF·V), which is comparable to tantalum capacitors and can work reliably below 55°C. 30 years.
1.3 Effect of temperature on leakage current
Under constant voltage, if the temperature gradually increases from room temperature, the leakage current will increase rapidly in the form of an exponential function. Generally, at the positive limit temperature (85°C), the leakage current of aluminum electrolytic capacitors is 2.5 to 3 times that of room temperature. For example, the leakage current coefficient of CD26/27 aluminum electrolytic capacitor is 0.03 uA/(uF·V) at 20℃, and its leakage current coefficient is 0.075 to 0.15 at the extreme temperature of 85℃. From an essential analysis, no matter what form of electrolytic capacitor, the leakage current has a common reason as the temperature rises, that is, the migration ability of impurity ions in the oxide film of the capacitor will sharply increase as the temperature rises.
Of course, each type of capacitor also has its own special reasons, and the degree of dependence is also different. For example, when using ammonium borate ethylene glycol/water system as the working electrolyte, at high temperatures, the oxide film is hydrated and changes part of its structural form, resulting in a sharp increase in leakage current. However, N,N-dimethylformamide is used as the The working electrolyte of a solvent will not have the destructive effect of hydration of the oxide film; in addition, if there are chloride ions inside the electrolyte or aluminum foil, the corrosion of the base metal and oxide film will increase at high temperatures, which will also lead to an increase in current, but this type of Unlike the increase in migration ability, the process will not change immediately as the temperature rises, but will take time to gradually have an impact.
In addition, since aluminum and tantalum oxide films also have a slow relaxation ion polarization form, at high temperatures, the interstitial ions in their structures can also participate in conduction and contribute to an increase in leakage current.
1.4 Effect of applied voltage size and duration
Leakage current increases with increasing applied voltage. It rises slowly when the voltage is lower than the rated voltage, and rises sharply when it slightly exceeds the rated voltage.
The reason why the leakage current increases with voltage can be understood as follows: when the applied voltage increases and the film thickness remains unchanged, the electric field intensity in the film will increase as the voltage increases. The relationship between electron current and field strength in the oxide film conforms to the following hyperbolic sine function relationship:
i= Asinh(BE) (7-83)
In the formula: A and B are relevant constants, and the value of A has an exponential relationship with temperature. Therefore the leakage current increases.
In addition, when the field strength reaches a certain value, the impurity ions that are weakly bound in the crystal lattice may break away from their bonds, thus increasing the number of participating conductive particles and increasing the leakage current.
In addition, the leakage current generally decreases slowly as the voltage application time increases, and finally reaches a steady state. There are three explanations for this phenomenon.
(1) Electrolytic capacitors have slow polarization, especially foil-type structures using liner paper. Due to their large capacitance, they show a large absorption current during the charging process, so it takes a long time to complete. .
(2) The oxide film of the electrolytic capacitor may be chemically or electrochemically damaged due to the presence of impurities, causing flaws or defects on the surface. Therefore, when voltage is applied, the electrolyte will repair part of the damaged film layer, causing the leakage current to decrease. The evidence is that for capacitors with a short interruption time, the relationship between the leakage current and the application time is not very obvious after the voltage is continued to be applied.
(3) During the use of electrolytic capacitors, after a period of time, the micropores and gaps in the film will be filled with oxygen, blocking the path between the electrolyte and the anode, so the leakage current is reduced. But when the voltage is removed, the oxygen in the holes is released. Therefore, the next time the voltage is continued to be applied, oxygen needs to be regenerated to plug the hole conductive path.
In order to measure the leakage current of aluminum electrolytic capacitors, it is generally specified that the measured current after 5 minutes is smaller than the actual leakage current. However, under production conditions, considering the decline of production efficiency and leakage current, the initial leakage current drops quickly, so enterprises can set a leakage current value of 30 seconds during production.
1.5 Effect of storage period and storage conditions
The longer the storage period of the aluminum electrolytic capacitor, the greater the possibility of the oxide film being damaged due to the role of the impurity metal ions and the aluminum foil forming a micro-battery and the hydration erosion of the aluminum oxide film by the working electrolyte with more water. The membrane may have a dissolving and damaging effect. This phenomenon is called disempowerment. Therefore, after a long period of storage, if the rated voltage is immediately connected, the leakage current will be very large, and it will decrease very slowly within a normal period of time, and the oxide film will not have time to repair. At the same time, the capacitor generates a lot of heat, and more parts of the oxide film are broken down, which may cause permanent damage. Therefore, generally for electrolytic capacitors that have been stored for a long time, the operating voltage should be gradually increased to the rated voltage so that the oxide film can be repaired in advance and normal operation can be restored.
In short, the size of the leakage current is the most direct indicator of the performance of the electrolytic capacitor and the suitability of the process and the degree of civilized production. Impurities in any raw materials and pollution during operation (dust, sweat, etc.) may lead to uneven oxidation growth. And there are defects on the film, and the main part of the steady-state leakage current, the electron current, is carried out through the defects and the edge of the foil.