Supercapacitors Aqueous Electrolyte
Supercapacitors aqueous electrolyte have high conductivity and low impedance. Due to the small molecular diameter of the electrolyte, it is easy to fully immerse into the micropores and make full use of its surface area. Compared with their organic counterparts, supercapacitors aqueous electrolyte have advantages in terms of affordability, electrical conductivity, heat capacity, and environmental impact. Their conductivity is at least an order of magnitude higher than that of organic and ionic liquid electrolytes, and supercapacitors aqueous electrolyte are inexpensive and do not require special conditions in the laboratory, making them easier to handle and greatly simplifying the fabrication and assembly of supercapacitors. So far, supercapacitors aqueous electrolyte are widely used in conventional and novel electrochemical supercapacitors. In the literature on supercapacitor electrolytes published in 2004, 84.8% of the electrolytes were supercapacitors aqueous electrolyte.
Supercapacitors aqueous electrolyte have been used in batteries for a long time. In 1799, Alessandro Volta developed the first chemical battery using sodium chloride solution as the electrolyte, commonly known as the “Volta” battery. In the 1860s, French Leclanche invented the Leclanche battery (carbon-zinc dry battery), which used ammonium chloride aqueous solution as the electrolyte. In addition to traditional batteries, in the rapid development of lithium-ion battery research in recent years, lithium-ion batteries use aqueous electrolytes, which can avoid the common safety hazards of organic electrolytes. However, the cycle life of lithium-ion batteries is relatively short for batteries using aqueous solution as the electrolyte, and the battery storage capacity is generally less than 50% after 100 cycles. Even if the electrode material is replaced, it is difficult to improve its cycle life. The latest related research has revealed the mechanism of capacity decay of aqueous lithium-ion batteries during cycling: the negative electrode material in the discharged state reacts with H2O and O2, regardless of the pH value of the solution. For typical mesoporous carbon electrode supercapacitors, the specific capacitance and energy density in supercapacitors aqueous electrolyte range from 100-200 F·g-1 and 10-50 W·h·kg-1.
The supercapacitors aqueous electrolyte solution in a supercapacitor can be any salt, acid, base or a combination thereof, but must not react with the electrode material. Among them, H2SO4, KOH, LiOH, Na2SO4, etc. are the most representative and commonly used supercapacitors aqueous electrolyte for supercapacitors. The selection criteria of supercapacitors aqueous electrolyte usually consider the size of hydrated cations and anions, as well as the mobility of ions (Table 5-1), which not only affects the ionic conductivity, but also affects the specific capacitance value. The capacitor and the degree of corrosion of the electrolyte to the electrode should be considered . The main disadvantage of supercapacitors aqueous electrolyte is that the electrochemical window of capacitors is relatively narrow due to the constraints of water decomposition. The hydrogen evolution reaction occurs at the negative electrode potential at about 0V, and the oxygen evolution reaction occurs at the positive electrode potential at about 1.23V. Therefore, the battery voltage of an electrochemical supercapacitor is about 1.23V. The gas generation can cause damage to the supercapacitor, reducing its performance and endangering safety. To avoid gas generation, the voltage of electrochemical supercapacitors with aqueous electrolytes is limited to around 1.0 V. Table 5-2 lists typical supercapacitors aqueous electrolyte electrochemical supercapacitors and their properties. It can be seen from the table that for acidic and alkaline electrolytes, no matter what electrode material is used, the voltage of supercapacitors is limited within 1.3V; for neutral electrolytes, the highest voltage of supercapacitors can reach 2.2V. In addition, electrochemical supercapacitors using aqueous electrolytes also have limitations on the operating temperature, which needs to be controlled above the freezing point and below the boiling point of water.
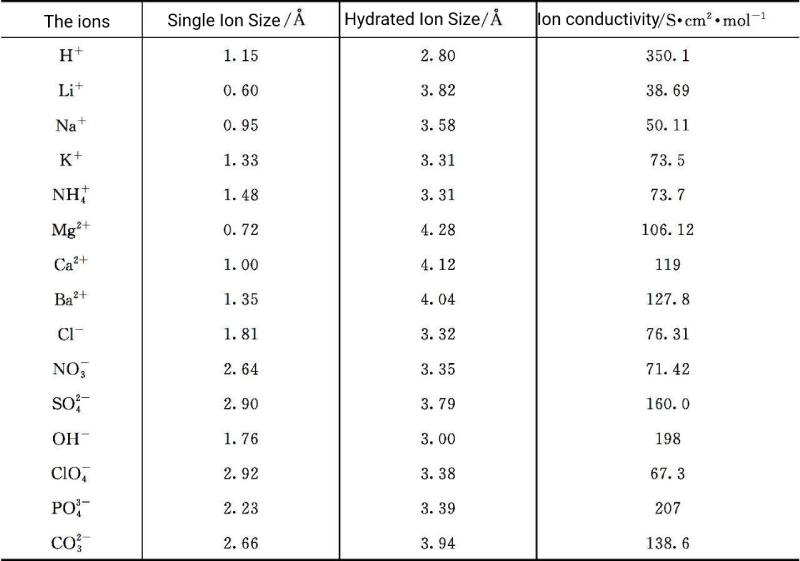
Table 5-1 Size of Single Ion and Hydrated Ion, and Values of Ionic Conductivity
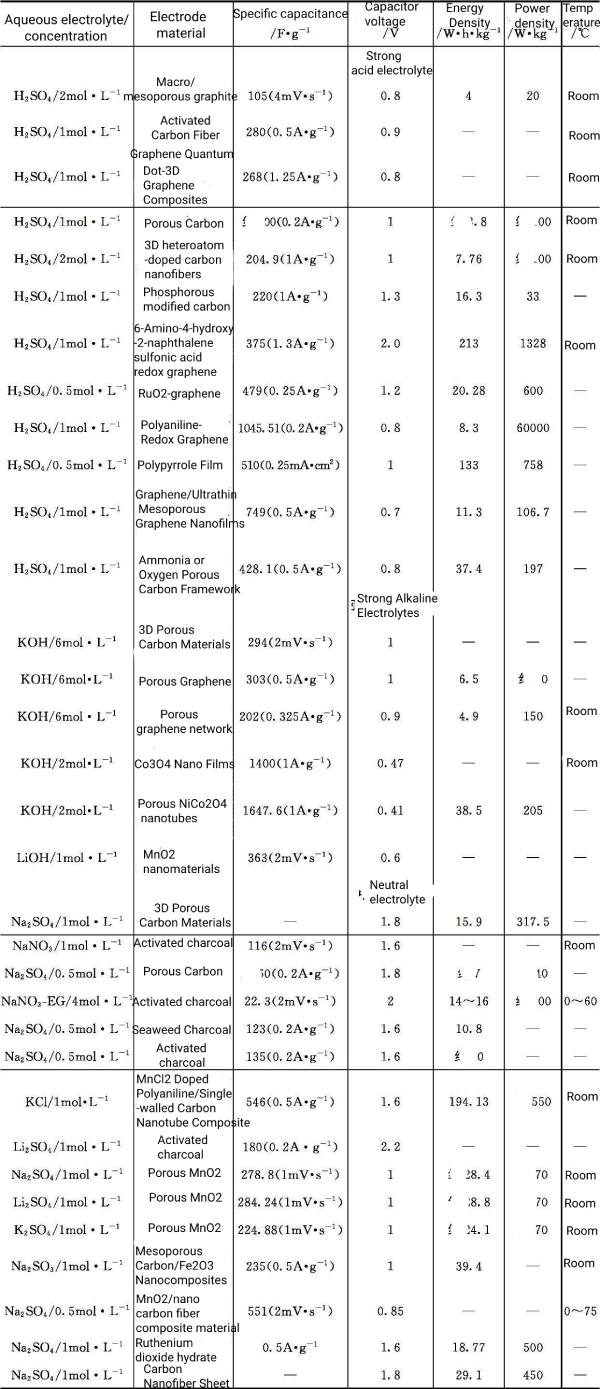
Table 5-2 Supercapacitors Based on Aqueous Electrolytes and Their Performance
1 Acidic aqueous electrolyte
Strong acid aqueous solution has the characteristics of high conductivity, high ion concentration, low internal resistance and low equivalent series resistance, so it is often used as the electrolyte of electrochemical supercapacitors. Among many acid electrolytes, H2SO4 electrolyte is the most commonly used because of its ultra-high conductivity (at 25 ℃, the conductivity of 1.0mol · L-1 H2SO4 is 0.8S · cm-1). The magnitude of conductivity mainly depends on the concentration of H2SO4. If the concentration is too low or too high, it can lead to a decrease in the ionic conductivity of the electrolyte. At 25 ℃, the ionic conductivity of H2SO4 reaches its maximum at 1.0mol · L-1, so most researchers use 1.0mol · L-1 H2SO4 as an electrolyte solution, especially for electrochemical capacitors using carbon based electrode materials.
1.1 Electrochemical double layer capacitors
Most studies found that the specific capacitance of the electric double layer capacitor using sulfuric acid electrolyte is larger than that using neutral electrolyte. In addition, because of the high ionic conductivity of H2SO4, the equivalent series resistance of the electrochemical supercapacitor when H2SO4 is used as electrolyte is lower than that when neutral solution is used as electrolyte . Research has found that the specific capacitance of activated carbon increases with the increase of electrolyte conductivity, which can be explained by considering the ion mobility closely related to electrolyte conductivity. In the past few years, reports have shown that the specific capacitance of double layer capacitors with strongly acidic electrolytes such as H2SO4 ranges from approximately 100 to 300F · g-1, which is much higher than that of capacitors with organic electrolytes as electrolytes.
1.2 Pseudocapacitance is a container
Aqueous electrolytes are not only widely used in double layer capacitors, but also in pseudo capacitor capacitors. When carbon based materials are used as electrodes and H2SO4 aqueous solution is used as electrolyte, in addition to generating double layer capacitance, there are also some Faraday quasi capacitors (pseudo capacitors). This type of pseudocapacitance is generated by the oxidation-reduction reaction of functional groups on the surface of the electrode material. By introducing heteroatoms (such as oxygen, nitrogen, phosphorus, etc.) to the surface of the electrode material, some surface functional groups can further enhance this type of pseudocapacitance. It is worth noting that the functional groups on the surface of the material undergo different reactions in different electrolytes, so the properties of the electrolyte have a significant impact on the pseudocapacitance performance of carbon based electrode materials.
For example, materials with quinone functional groups on their surface participate in the redox reaction of protons in acidic electrolytes to generate pseudocapacitance, and the reaction process is shown below. However, in alkaline electrolytes, this reaction is almost impossible to occur. Therefore, selecting an appropriate electrolyte to maximize the generation of functional groups on the surface of the electrode material Pseudocapacitance is crucial for optimizing the performance of supercapacitors. In addition, due to the fact that electrode materials containing functional groups are more prone to degradation in supercapacitors aqueous electrolyte, the cycle life of pseudo capacitor capacitors is shorter than that of double layer capacitors. One way to solve this problem is to introduce certain surface functional groups (such as phosphorus containing groups) into carbon materials, which can to some extent improve the stability of electrode materials in aqueous electrolytes.
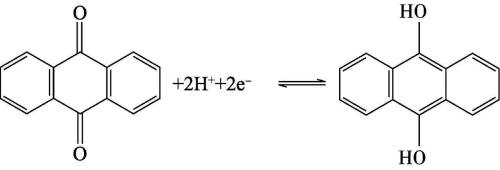
In addition, electrode materials for pseudo capacitor capacitors can also use non carbon based materials such as metal oxides, sulfides, and conductive polymers. In the presence of supercapacitors aqueous electrolyte, the theoretical capacitance when using these materials as electrodes is higher than that of carbon based materials. However, these electrode materials are very sensitive to the type and pH value of the electrolyte, and are extremely unstable in acidic electrolyte aqueous solutions. Only a small amount of non carbon based materials are suitable for pseudo capacitor capacitors in strongly acidic electrolytes. RuO2, as a pseudocapacitive electrode material suitable for H2SO4 electrolyte, has been widely studied and reported. When amorphous RuO2 is used as an electrode material, the capacitance value can reach up to 1000F · g-1, which may be due to the ease with which proton hydrogen can enter the amorphous structure. However, the high cost and limited sources of metal Ru have limited its commercial development, so researchers have attempted to use some materials with better performance in strong acid electrolytes, such as α- MoO3) replaces RuO2.
1.3 Hybrid Capacitors
To increase the energy density of aqueous electrolyte electrochemical supercapacitors, the development of hybrid supercapacitors with a wider voltage window has received extensive attention. For example, when symmetric electrochemical supercapacitors with the same electrode material use supercapacitors aqueous electrolyte such as H2SO4 and KOH, the maximum voltage of the capacitor is limited by the gas evolution reaction. However, if an electrochemical supercapacitor with an asymmetric configuration (with different anode and cathode materials) is adopted, the resulting electrochemical supercapacitor has a wide operating potential window even in supercapacitors aqueous electrolyte solutions.
In electrochemical supercapacitors, the use of two different electrode materials complements the potential electrochemical window. The carbon-based negative electrode undergoes a hydrogen evolution reaction at a higher potential, and the battery positive electrode (PbO2) or pseudocapacitive electrode (RuO2) undergoes an oxygen evolution reaction. This could provide an operating voltage window for electrochemical supercapacitors beyond the thermal limit of supercapacitors aqueous electrolyte. So far, electrochemical supercapacitors with asymmetric configurations using strongly acidic aqueous solutions as electrolytes (e.g. carbon/lead dioxide, carbon/ruthenium dioxide, carbon/conducting polymers, carbon-based materials of different qualities or properties electrodes) have been measured and the feasibility of this capacitor has been demonstrated.
For example, in the case of the same concentration of H2SO4 as the electrolyte, the energy density of carbon and lead dioxide hybrid electrochemical supercapacitors can reach 25-30 W h kg-1, which is much higher than that of carbon-based double-layer supercapacitors. Density (3-6W·h·kg-1). However, in the H2SO4 electrolyte, the stability of the lead dioxide electrode is poor. Pere et al. found that the H2SO4 electrolyte with a concentration of 1mol L-1 would damage the structure of the lead dioxide nanowires during the electrochemical cycle, resulting in poor electrochemical cycle performance of the capacitor. To address this issue, they investigated the use of methanesulfonic acid (CH3SO3H) and lead methanesulfonate as electrolyte substitutes, in which case the redox process on the PbO2 electrode changed from solid/solid coupling in the H2SO4 electrolyte to that in methanesulfonic acid Solid/solvated ions in base electrolyte [3%].
During the whole discharge process, PbO2 is reduced to Pb2+ into solution. During the entire charging process, Pb2+ in the solution is oxidized to PbO2, causing electrodeposition on the electrode surface. Therefore, the H2SO4 electrolyte does not limit the acidification of PbO2, but better realizes the cycle of this reaction. Although this can increase the energy density of electrochemical capacitors, it will affect the power density and cycle life of capacitors. To solve this problem, asymmetric hybrid electrochemical capacitors that combine pseudocapacitor electrode materials with carbon-based materials, such as anthraquinone-modified Carbon/ruthenium oxide, carbon conductive polymers, or carbon/carbon with different surface functional groups have been continuously developed.
It should be noted that in addition to H2SO4, common acidic supercapacitors aqueous electrolyte include perchloric acid, hexafluorophosphoric acid, tetrafluoroboric acid, etc. When these acidic aqueous solutions are used as electrolytes, they are highly corrosive and metal materials cannot be used as current collectors. Squeezing a supercapacitor can cause the electrolyte to leak, which can lead to more corrosion. In addition to the safety of the electrolyte used, the self-discharge phenomenon inside the supercapacitor also needs to be considered, especially the generation of metal ions and oxygen when there is pollution. Therefore, other acidic aqueous solutions can be considered as electrolytes for supercapacitors. Daniel Bé langer et al used 4.0mol L-1 HBF4 aqueous solution as the electrolyte in polyaniline electrochemical capacitors, the specific energy of the capacitor was 2.7W h kg-1, and the specific power was 1.0kW kg-1. Shuping et al. studied the ordered mesoporous carbon material BOMC-5 prepared by doping 5% boric acid in the H2SO4 electrolyte, and the mass specific electric value of the supercapacitor can reach 140.9F·g-1.
2 Alkaline aqueous electrolyte
Alkaline aqueous electrolyte is one of the most widely used supercapacitors aqueous electrolyte in practical applications. Compared with strongly acidic electrolytes, some low-cost metallic materials such as Ni can be used as current collectors for electrochemical capacitors. Among alkaline aqueous electrolyte, the most commonly used electrolyte is KOH aqueous solution. The concentration of KOH aqueous solution is generally 1-6 mol·L-1. When carbon-based materials are used as electrodes, the concentration of KOH is generally 6 mol L-1, while when metal oxides are used as electrodes, the concentration of KOH is usually 1 mol L-1. Besides aqueous KOH, aqueous LiOH or NaOH are also used as electrolytes in some supercapacitors, and these alkaline electrolytes can be used in carbon-based electric double layer capacitors, pseudocapacitive capacitors [e.g. Ni(OH)2 and CO3O4], and Hybrid electrochemical supercapacitors. However, when an alkaline aqueous solution is used as the electrolyte, there is an “alkali climb phenomenon”, which makes the sealing problem of supercapacitors difficult.
2.1 Double layer capacitors
In literature reports, the specific capacity and energy density values of double layer supercapacitors with KOH as the electrolyte are similar to those of H2SO4 as the electrolyte. In addition to using strong acidic electrolytes, a large number of studies have reported the use of alkaline electrolytes to increase the specific capacitance of capacitors or expand the operating voltage window, thereby improving the energy density of double layer capacitors. These developments can be summarized as follows:
① Improving the capacitance of carbon based electrode materials by introducing pseudocapacitance;
② Develop pseudo capacitive materials with high specific capacitance;
③ Exploring composite materials that combine carbon based materials and pseudo capacitive materials;
④ The design of asymmetric electrochemical supercapacitors has increased the working voltage window of alkaline electrolytes.
2.2 Pseudocapacitive capacitors
The pseudo capacitance of carbon based electrode materials is generated by the Faraday interaction between functional groups on the carbon surface and ions in the electrolyte. Research has found that KOH electrolytes are more suitable for nitrogen doped carbon electrode materials, indicating that pseudocapacitance is related to the type of electrolyte and pH value. Wang et al. reported on the super properties of porous carbon co doped with phosphorus and nitrogen Grade capacitors, compared to phosphorus free electrode materials, exhibit higher specific capacitance, wider potential windows, and higher stability, further improving the performance of electrochemical supercapacitors. In addition, we noticed that hydrogen can be stored through the polarization of the negative electrode and the potential is lower than the thermodynamic reduction potential value of water, which is also related to the type of electrolyte. It is more likely to occur under the condition of alkaline electrolyte.
In alkaline electrolytes, some transition metals (such as NiOx, CoOx, MnO2, and NiCo2O4), hydroxides (such as Ni (OH) 2, Co (OH) 2), sulfides (such as cobalt sulfide), and nitrides (such as alum nitride) have been widely studied as electrode materials due to their high theoretical capacitance values. The interaction between ions in the electrolyte and electrode materials plays an important role in the pseudocapacitance behavior of these materials, and these pseudocapacitors.
The charge storage mechanism of electrode materials usually includes the process of adsorption/desorption or the insertion/removal of electrolyte ions into/out of the electrode material. For example, Feng et al. prepared CO3O4 nanofilms below 3nm and obtained a specific capacitance of up to 1400F · g-1 in 2mol · L-1 KOH electrolyte. Mefford et al. proposed in recent research the anionic insertion charge storage mechanism of LaMnO3 structured pseudocapacitive electrodes in KOH electrolytes, providing a new approach for obtaining high capacitance pseudocapacitive materials. It should be noted that there may be an electrochemical reaction between the electrode material and the electrolyte, which has a significant impact on the pseudocapacitive behavior. For example, some metal sulfides such as CoSx and NiS exhibit poor pseudocapacitance performance in KOH electrolytes. However, when they are converted into new electroactive substances CO(OH)2 and Ni (OH)2 in KOH electrolyte, the pseudocapacitance increases significantly.
Usually, the properties of electrolytes, such as ion type, concentration, and operating temperature, can affect the performance of electrochemical supercapacitors. The results show that the concentration of alkaline electrolyte has influence on equivalent series resistance, specific capacitance and oxygen evolution reaction value. The disadvantage of using concentrated alkaline electrolytes is that the electrode surface is prone to corrosion, and the electrode material is easily detached from the substrate. Therefore, it is necessary to optimize the electrolyte concentration of the entire electrochemical supercapacitor.
The increase of electrolyte temperature usually enhances the ion diffusion process, leading to the decrease of equivalent series resistance and the increase of capacitance. However, with the increase of temperature, the oxygen evolution reaction on the positive electrode intensifies, leading to the decrease of the initial potential of oxygen evolution reaction. In addition, in KOH electrolytes, increasing temperature can cause oxidation on the electrode surface, leading to material degradation, thereby reducing the cyclic stability of activated carbon.
Due to the fact that the intercalation and de intercalation of alkaline electrolyte ions generally involve pseudocapacitive materials, the size of non solvated ions may have a significant impact on pseudocapacitive behavior. Generally speaking, the influence of ionic electrolytes on the performance of electrochemical supercapacitors is relatively complex. For example, some researchers have found that the specific capacitance of electrochemical supercapacitors is higher when MnO2 uses LiOH as the electrolyte than when KOH or NaOH is used as the electrolyte. Researchers attribute this to the relatively easy insertion/detachment of Li+, as Li+ has a smaller radius compared to K+ or Na+. Inamdar et al. found that the specific capacitance of NiO in NaOH electrolyte is about twice that in KOH, which is attributed to the high embedding rate of sodium ions on the electrode material surface. However, when Co2P2O7, MnF2O4, and Bi2WO6 are used as electrode materials, the specific capacitance value of supercapacitors using KOH electrolyte is higher than that using NaOH or LiOH electrolyte. However, due to limited comparative research on various alkaline electrolytes, it is currently unclear whether this phenomenon is related to the type of electrolyte, electrode material type or preparation process, and the obtained material structure.
As mentioned above, pseudocapacitive materials have poorer cyclic stability compared to non pseudocapacitive materials, and this instability may be caused by the continuous intercalation/de intercalation of ions in alkaline electrolytes. In addition, the dissolution of electrode materials in alkaline electrolytes may also lead to a decrease in capacitance performance after long-term charge/discharge cycles.
Similar to the research on acidic electrolyte electrochemical supercapacitors, how to expand the working voltage window of alkaline electrolyte supercapacitors to significantly improve their energy density is the current research trend. For symmetrical supercapacitors, the electrode stability under a larger potential window or the inhibition of electrolyte side reactions can usually be achieved by modifying the electrode material, thereby improving the performance of the capacitor.
2.3 Hybrid Capacitors
According to reports, in order to increase the energy density of capacitors, a series of asymmetric supercapacitors using alkaline electrolytes and having a wide potential window have been developed. For this asymmetric electrochemical supercapacitor, the electrode materials for the positive and negative electrodes are different.
The positive electrode of an asymmetric supercapacitor is a battery-type [such as Ni (OH)2 ] or pseudocapacitive (such as RuO2) electrode material that stores charges through Faradaic reactions, and the negative electrode is a carbon-based material, where the charge is mainly in the form of an electric double layer. store. KOH electrolyte can effectively increase the operating voltage of these asymmetric electrochemical supercapacitors, for example, the voltage of carbon/Ni(OH)2 is 1.7 V, the voltage of carbon/Co(OH)2, carbon/Co3O4) and carbon/Co9S8 Between 1.4 ~ 1.6V, the voltage of carbon/Ni3S2 is 1.6V, and the voltage of carbon/RuO2-TiO2 is 1.4V. Due to the use of large operating voltage window and high-capacity Faraday-type electrode materials, the energy density of most of these electrochemical supercapacitors ranges from 20 to 40 W h kg-1, and some can even reach 140 W h kg-1. 1, which is comparable to a rechargeable potassium-ion battery.
However, due to the use of Faraday-type electrodes, the cycle stability of these asymmetric electrochemical supercapacitors is usually much lower than that of electric double layer electrochemical supercapacitors after 1000–5000 cycles, and some asymmetric electrochemical supercapacitors have a specific capacitance loss of more than 10%, besides, the charging and discharging process of these asymmetric electrochemical supercapacitors is significantly slower than that of electric double layer supercapacitors.
3 Neutral supercapacitors aqueous electrolyte
In addition to acidic and alkaline electrolytes, neutral electrolytes have also been widely used in supercapacitors, because neutral electrolytes are safe, non-corrosive, and can use various current collectors. The capacitor assembly process is simple and economical, and has relatively Large electrochemical window, in addition, the performance of the capacitor is relatively stable, and the pollution to the environment is small. Typical neutral electrolyte salts mainly include potassium salts (LiCI, Li2SO4 and LiCIO4), potassium salts (KCI, K2SO4 and KNO3), sodium salts (NaCl, Na2SO4 and NaNO3, calcium salts [Ca (NO3) 2] and magnesium salts ( MgSO4). Among these neutral electrolyte solutions, Na2SO4 is the most commonly used neutral electrolyte and is a promising electrolyte for many pseudocapacitive materials, especially those based on MnO2. Although there are some The research on neutral electrolytes is applied to electric double layer capacitors, but the most important ones are applied to eagle capacitance capacitors and hybrid electrochemical supercapacitors.
3.1 Electric Double Layer Supercapacitor
Electric double layer capacitors usually use activated carbon electrode materials with a high specific surface area, and the mechanism for storing charges is the reversible adsorption/desorption process of ions at the electrode/electrolyte interface.
Through comparative studies, it is found that the specific capacitance of neutral electrolyte electric double layer capacitors is lower than that of H2SO4 electrolyte or KOH electrolyte, and the equivalent series resistance of electrochemical supercapacitors using neutral electrolytes is lower than that of H2SO4 or KOH, However, carbon-based electrochemical supercapacitors with neutral electrolytes may yield higher operating voltages due to the increased electrochemically stable potential windows (ESPWs) due to the increased electrolyte stability compared with acidic and alkaline electrolytes. Moreover, neutral electrolytes have lower H+ and OH- concentrations than acidic and alkaline electrolytes, so hydrogen evolution and oxygen evolution reactions will get higher potentials, which will increase the electrochemical stability window.
For example, Demarconnay et al. found that a symmetric activated carbon-based electrochemical supercapacitor using 0.5mol L-1 Na2SO4 electrolyte maintained good capacitor performance after 10,000 charge/discharge cycles at a high battery voltage of 1.6V. Zhao et al. used nitrogen and oxygen doped carbon nanofiber electrodes and used 1mol L-1Na2SO4 as the electrolyte to further increase the voltage of the electrochemical supercapacitor to 1.8V, and the power density reached 29.1W at an energy density of 450W kg-1. h·kg-1. When a carbon-based symmetric electrochemical supercapacitor uses 1mol L-1Li2SO4 as the electrolyte, the obtained operating voltage is 2.2V, and the electrochemical supercapacitor still maintains the original capacitance characteristics after 15000 cycles. In order to investigate the carbon-based degradation of electrochemical supercapacitors with Li2SO4 as electrolyte under high-voltage operation, Ratajczak et al. conducted some accelerated aging tests, and they found that due to the oxidation of carbon electrode materials, gases (such as CO2 and CO ) may start to discharge at battery voltages higher than 1.5V.
It is thus concluded that the safe voltage of carbon-based electrochemical supercapacitors with 1 mol L-1 Li2SO4 aqueous solution is 1.5 V, which is lower than the capacitor voltage in other literatures. It is worth noting that in this study, the current current collector was stainless steel instead of gold as reported in other studies, which may be the reason for the different results. The operating voltage obtained by electrochemical supercapacitors based on neutral electrolytes is significantly higher than that of KOH and H2SO4 electrolytes (generally, the operating voltage of carbon-based symmetric supercapacitors is 0.8 ~ 1 V), while neutral electrolytes are less corrosive than strongly acidic and alkaline electrolytes. , the electrochemical supercapacitor with neutral solution as the electrolyte and symmetrical carbon as the electrode material is considered to be one of the most promising capacitors due to its low impact on the environment and high energy density.
For neutral electrolytes, the availability of high-concentration salt solutions is an important issue.
problem, this is not a problem for acidic and alkaline electrolytes because they can achieve high concentration (for example, the concentration of KOH electrolyte is usually 6mol L-1), in general, high concentration electrolytes are used in electrochemical capacitors to ensure high performance capacitors. However, some salts (such as K2SO4) cannot achieve such high concentrations, especially at lower temperatures. In fact, the effect of neutral electrolytes on the performance of electrochemical supercapacitors also depends on the type of electrolyte.
In order to understand the effect of different ions on the performance of carbon-based electrochemical supercapacitors, some comparative studies have been performed on neutral electrolytes with different salts, however, some results in this regard are still controversial. For example, alkali metal salt sulfuric acid electrolytes include Li2SO4, Na2SO4, and K2SO4. Some studies have found that the capacitance values of electrochemical capacitors follow a specific order of Li2SO4>Na2SO4>K2SO4, but some studies have not shown such results, and others Factors, such as material preparation method and measurement conditions, voltage sweep rate/discharge rate, may also affect the experimental results, and in this regard, further work may be needed to further clarify the effect of salt on the performance of electrochemical supercapacitors. For the equivalent series resistance, the equivalent series resistance increases with the increase of electrolyte resistivity Li2SO4>Na2SO4>K2SO4, while the order of power density and rate performance is Li2SO4<Na2SO4<K2SO4.
Regarding the effect of anions on neutral electrolytes, the researchers found that for electrolytes with the same cations and concentrations, changing anions from SO2-/4 to CI- can increase the specific capacitance of a supercapacitor, because relative to SO2-/4, CI- is smaller in size. Recently, Gao et al. have studied some new electrolytes as aqueous neutral electrolytes for electrochemical double layer capacitors such as potassium salts, sodium salts and potassium salts (Li-Siw, Na-SiW and K-SiW) of tungstosilicate, and CI-, SO2-/4, or NO-/3, these electrolytes have higher ionic conductivity compared to anions, because the higher the number of cations, the higher the ion mobility of Keggin-type anions, for those with these neutral For electrolytic carbon-based electrochemical double-layer capacitors, the capacitor voltage has reached 1.5V.
3.2 Pseudocapacitive capacitors
In neutral electrolytes, MnO-/2, and V2O-/5, base electrode materials have proven to be promising pseudocapacitive materials in electrochemical supercapacitors, and MnO2 is by far the most widely used in neutral electrolytes The studied pseudocapacitive electrode material, when MnO2 is used as the electrode material, is accompanied by the surface adsorption/desorption or intercalation/deintercalation of electrolyte cations M+ (such as K+, Na+, and Li+) as well as cations (H+) during charge/discharge , the valence state of Mn can vary between 3 and 4.
Since the electrolyte ions are directly involved in the charge storage process, which stores charges through the redox reaction of the electrolyte on the electrode surface, the properties of the neutral electrolyte have a significant impact on the performance of the pseudocapacitive capacitor.
Some other factors of the neutral electrolyte, such as pH value, cation and anion species, salt concentration, additives, and solution temperature, all have an impact on the performance of electrochemical supercapacitors. For the types of cations, various alkaline metal or alkaline earth metal cations have different ion sizes and hydrated ion sizes, and thus have different diffusion coefficients and ion conductivities, which have an impact on the specific capacitance and equivalent series resistance of electrochemical supercapacitors. large effect. However, the dependence of the specific capacitance on the cation species is not well understood due to variations in the preparation method and electrode material structure.
The order of specific capacitance values and corresponding specific energy and power density values for mesoporous MnO2-based electrodes in neutral electrolytes has been reported: Li2SO4Na2SO4>K2SO4, which seems to be related to the single (undissolved) ions of these alkali metal ions The size of the ion is related, such as Li+>Na+>K+, indicating that the smaller ion size is beneficial to improve the specific capacitance of the capacitor. In contrast, studies have shown that when manganese dioxide is used as an electrode material, sodium salts as electrolytes (such as sodium sulfate and NaCI) have higher specific capacitances than lithium and potassium salts of capacitors. When KxMnO2·nH2O is used as electrode material, K2SO4 salt can produce higher capacitance than Na2SO4 and Li2SO4 salt. Wen et al found that when using three different electrolytes (KCIO4, NaCIO. performance.
However, the mass specific capacitance value of an electrochemical supercapacitor also depends on other factors such as structure (e.g. d-MnO2 or a-MnO2), morphology, preparation method, and the amount of electrode material, therefore, if the mass specific capacitance is used for comparison It is not easy to identify the effect of cation species on the capacitance of electrochemical supercapacitors. For example, different preparation methods can often lead to electrode materials with different pore structures, which may also affect the diffusion path of electrolyte ions and complicate the whole process.
In this regard, further research using electrode materials with a certain specific surface area may help to understand the influence of cations on the intrinsic capacitance of electrode materials, and, since the intercalation/deintercalation of cations involves MnO2, the structure of the base electrode material, Therefore, the scan rate of cyclic voltammetry or the charge/discharge current density may also have a significant impact on the obtained specific capacitance value. The intercalation/deintercalation rate of ions is considered to be determined by the charge/discharge process, so reducing the scan speed or charge/discharge rate may benefit the intercalation/deintercalation process, in which case the size of individual ions is related to the intercalation process related, may play a leading role. For example, electrolytes containing Li+ will benefit the performance of electrochemical supercapacitors because the smaller size of Li+ may facilitate ion intercalation/deintercalation.
In addition to alkaline metal ions, the effect of alkaline earth cations on the performance of electrochemical supercapacitors was also investigated. For example, using divalent cations (Mg2+, Ca2+, and Ba2+) instead of monovalent cations (Li+, Na+, and k+) will double the specific capacitance of an electrochemical supercapacitor using MnO2 as the electrode material. The reasons for this result are: When a divalent alkali metal cation is embedded in MnO2, it can balance the valence change of two Mn ions from 4 to 5, while a monovalent alkali metal cation can only balance the valence change of one Mn ion.
For anions, Boisset et al. used birnessite-type or potash-type MnO2 as electrode materials, studied the influence of anions on the performance of neutral aqueous electrolytes containing different potassium salts, and found that in each potassium salt electrolyte The basicity and volume of the anion are two key parameters controlling the oxidation and reaction current electrochemical performance of MnO2 electrodes.
Regarding the effect of temperature, unlike carbonaceous materials that are usually stable in a wide temperature range, it was found that MnO2 electrode materials undergo some structural changes during charge/discharge at high temperatures, which may negatively affect cycle stability. Influence. Therefore, the effect of electrolyte temperature on the pseudocapacitive behavior, especially on the cycling stability of MnO2-based electrochemical supercapacitors, is considered to be one of the important issues.
Regarding the cycle life of electrochemical supercapacitors with neutral electrolytes, since the charge storage process of most pseudocapacitive materials (such as MnO2) involves the insertion/deintercalation of ions in the electrolyte, during repeated charge/discharge processes, the electrode Changes in material structure may seriously affect the cycle life of electrochemical supercapacitors.
In addition to MnO2, the effect of electrolytes on other pseudocapacitive metal oxides (such as V2O5, Fe3O4, SnO2, ZnO, and RuO2) and conducting polymers has also been reported. In fact, the role of the electrolyte depends largely on the type of electrode material, for example, RuO2 electrodes are usually applied in acidic electrolytes, but RuO2-based electrochemical capacitors with neutral aqueous electrolytes (i.e. 1mol L-1 Na2SO4 A high working voltage of 1.6V can also be achieved, and the energy density is 19W·h·kg-1 when the power density is 500W·kg-1. In addition, due to the milder pH conditions, neutral electrolytes are used for various conductive polymer-based Electrochemical supercapacitors are also beneficial.
3.3 Mixed electrolytes
Neutral electrolytes are also widely used in asymmetric electrochemical capacitors with larger operating voltages and thus higher energy densities. Recently reported the use of graphite coated with gel polymer electrolyte (GPE) and LISICON As the negative electrode, LiFePO4 as the positive electrode, the electrolyte is a potassium ion battery with water-soluble electrolyte, and a simple LISICON film composed of Li2O-Al2O3-SiO2-P2O5-TiO2-GeO2 is fixed on the gel polymer as a solid separator to isolate water And only allow Li+ to pass through. The mechanism of action of this potassium-ion battery is shown in Figure 5-1. When the battery is charged, Li+ will be deintercalated from the structure of LiFePO4, then sequentially pass through the aqueous LISICON film and finally through the gel polymer electrolyte. During the charging process, Lit will eventually intercalate into graphite. During discharge, the opposite process takes place.
The average discharge voltage of the LISICON thin-film aqueous potassium-ion battery is as high as 3.1V, and its specific energy value is 258W h kg-1. The average discharge of the potassium-ion battery with Li metal coated with LISICON thin film as the negative electrode and LiMn2O4 as the positive electrode The voltage can be as high as 4.0V, and its specific energy value is 446W·h·kg-1. In similar aqueous capacitors, metallic magnesium has been considered as a substitute for metallic potassium. The Grignard reagent of PhMgBr was used to stabilize the Mg metal anode, while the cathode was still made of LiFePO4 to construct a hybrid rechargeable aqueous capacitor of Mo metal and Li ions. The specific energy of this hybrid capacitor can reach 245W·h·kg-1. Same as the LiTi2(PO4)3/Li2SO4/LiFePO4 aqueous solution capacitors mentioned above, in these LISICON film-based Mg metal and Li ion hybrid aqueous capacitors, Li2SO4 aqueous solution is used as the electrolyte, except that it has high ionic conductivity and the Li2SO4 aqueous electrolyte does not change Properties of LISICON thin films as solid electrolytes.
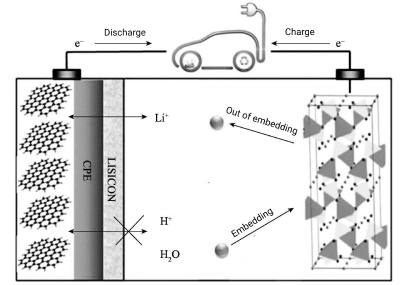
Figure 5-1 Lithium battery working
Note: GPE and LISICON are used as the negative electrode, LiFePO4 is used as the positive electrode, and the electrolyte is 0.5mol-L-1Li2SO4 aqueous solution
Despite few reports in the literature, the development and research of aqueous K-ion batteries are still ongoing to meet the demand for low-cost, high-safety electrochemical supercapacitor devices. Similar to the research on potassium-ion batteries, most research work on Na+ capacitors has also been carried out. Recent studies have shown that hollow K0.27MnO2 nanospheres can facilitate electron/ion transport in anions, resulting in a longer cycle and higher rate capability. By using hollow K0.27MnO2 nanospheres as negative electrodes, NaTi2 ( PO4) 3 is used as the positive electrode and 1.0mol L·1 Na2SO4 aqueous solution is used as the electrolyte to form a button-type supercapacitor. When the current is 150mA·g-1, its specific capacity can reach 84.9mA·h·g-1. ·g-1, its specific capacitance can still be maintained at 56.6mA·h·g-1. After 100 cycles, the specific capacitance of the capacitor is 200mA·h·g-1, and the capacity of the capacitor still maintains 83% of the initial value. It can be seen that the research on the electrolyte of sodium ion supercapacitors should be paid enough attention.
The pH value of the aqueous electrolyte will affect the performance of the electrode material, thereby affecting the performance of the supercapacitor. For example, the capacitive behavior of MnO2 under non-stoichiometric conditions. MnO2 is one of the most widely used electrode materials in supercapacitors. It only exhibits a rectangular-like cyclic voltammogram (CV) in neutral aqueous electrolytes, but a bell-shaped cyclic voltammogram (CV) in alkaline solutions. ). The reason for these apparent dynamic changes is that the redox reaction of MnO2 to MnOOH occurs on the positive electrode, which provides the eagle capacitance and makes its cyclic voltammogram rectangular. In a neutral electrolyte, MnO2 is outside the semiconductor state, resulting in a rectangular-like cyclic voltammogram. Since the solubility of MnOOH increases in concentrated alkali solution, MnO2 will be reduced to insulator Mn(OH) 2 in alkaline solution, and the dissolved manganese will be reduced to generate manganese ions at low voltage, which will eventually combine with OH- Insoluble Mn (OH )2 is formed. In this case, neutral electrolytes such as Li2SO4, Na2SO4, K2SO4, and KCI solutions are widely used in the research of supercapacitors based on MnO2 as electrode material. When using K2SO4 aqueous solution as the electrolyte, the specific energy of the supercapacitor can reach 17.6W·h·kg-1 when the specific power is 2kW·kg-1, and its electrochemical performance is better than that of the supercapacitor with Li2SO4 as the electrolyte. Recently, it was reported that with regard to cycle performance, an asymmetric supercapacitor composed of a-MnO2/CNT as the negative electrode, activated carbon as the positive electrode, and Na2SO4 as the electrolyte remained at the initial capacity after 2000 charge/discharge cycles at 50A g-1 77% of the value.
Most pseudocapacitive materials (such as MnO2) have high specific capacitance, but their potential window is limited. Therefore, if these electrode materials are used, the working voltage and energy density of symmetric electrochemical supercapacitors are limited. For symmetric electrochemical supercapacitors with manganese dioxide, in most cases, the battery voltage is about 1V, and the negative electrode is replaced by other electrode materials (such as activated carbon), which has a complementary window with manganese dioxide. By extending to negative voltages, it can Significantly increases the capacitor voltage.
Compared with the aforementioned asymmetric electrochemical supercapacitor positive electrodes using strongly acidic or strongly alkaline electrolytes [e.g. activated carbon/PbO2 and activated carbon/Ni(OH)2], the asymmetric activated carbon/manganese dioxide electrochemical supercapacitor Due to the pseudocapacitive behavior of MnO2 in neutral electrolyte, it has a good cycle life. In the early research, Hong et al. used neutral electrolytes in asymmetric activated carbon/MnO2 electrochemical supercapacitors, and made great contributions to the development of neutral electrolyte-based asymmetric electrochemical supercapacitors.
So far, various types of anode and cathode materials have been reported in neutral supercapacitors aqueous electrolyte (mainly sulfate electrolytes), and the operating voltage range of these asymmetric electrochemical supercapacitors can reach 1.8–2.0 V, higher than Asymmetric electrochemical supercapacitors with strongly acidic or alkaline electrolytes. Due to the increase of capacitor voltage, the energy density of most electrochemical supercapacitors can reach more than 20W h kg-1, some even reach 50W h-kg-1, these energy density values are equivalent to or even higher than those of organic electrolyte double electric layer capacitor. Therefore, these asymmetric electrochemical supercapacitors using neutral electrolytes are expected to replace commercial organic electrolyte EDLCs if the cycle life and rate performance of the capacitors can be further improved.
Recently, capacitor voltages up to 4 V have been reported in neutral electrolyte solutions such as Li2SO4 and LiCI using suitable cathode materials such as activated carbon and MnO2 combined with battery-type Li negative electrodes. Since metallic Li electrodes cannot directly contact supercapacitors aqueous electrolyte, water-stable multilayer Li can be used as negative electrodes (protected Li electrodes).
In summary, the use of neutral aqueous electrolytes in electrochemical supercapacitors can not only solve the corrosion problem, but also increase the operating voltage and thus increase the energy density. However, in order to improve the energy density and cycle life of capacitors, the performance of electrochemical supercapacitors with neutral electrolytes needs to be further improved.
4 Additives for supercapacitors aqueous electrolyte
Supercapacitors (SCs) are efficient and practical energy storage devices. In the past decades, different types of carbon-based materials, metal oxides, metal hydroxides, conducting polymers, and their various composites have been used as electrodes to enhance the energy performance of supercapacitors. In order to further improve the performance of electrochemical supercapacitors, some research groups have recently proposed another method, adding additives to the neutral electrolyte, such as introducing redox additives or mediators into the electrolyte, and the electrolyte (liquid and polymer) can pass through Redox reactions at the electrode/electrolyte interface to enhance the performance of supercapacitors. Compared with the preparation of some active electrode materials, this new technology has the main advantages of simple and safe preparation method and low cost.
Supercapacitors aqueous electrolyte is usually used as the ionic medium for charge storage in supercapacitors, but it cannot improve the specific capacitance of supercapacitors, while the performance of supercapacitors can be enhanced by using redox additives (individuals) or active materials. Electrolytes can be divided into the following three types:
1. Redox Additives – Liquid Electrolytes
2. Redox active liquid electrolyte
3. Redox Additive-Polymer Gel Electrolyte
The above-mentioned electrolyte active agents are a new field, and their addition can effectively improve the capacitance or energy density of supercapacitors. Compared with the preparation of new electrode materials, the method of adding active agents is simple, safe, efficient and low in cost, has good redox reversibility, is non-toxic, and is suitable for large-scale production.
4.1 Redox Additives—Liquid Electrolytes
The electrolyte formed by adding redox additives or compounds directly into the electrolyte is called a redox additive electrolyte or a mediator electrolyte. For example, hydroquinone is added to H2SO4, hydroquinone is an additive, and H2SO4 is an electrolyte. These redox additives or compounds directly participate in the redox reaction of electron transfer. Due to the formation of surface pseudocapacitance at the electrode electrolyte interface, the electric The performance of chemical capacitors has been improved. Different materials such as hydroquinone and KI are used as redox active additives added to conventional electrolytes (KOH, H2SO4, etc.), and the currently reported ones are listed in Table 5-3.
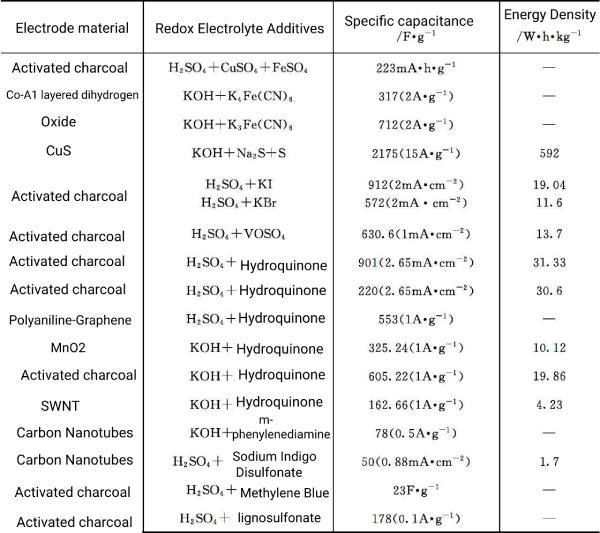
Table 5-3 Properties of capacitors after adding redox additives
For example, Komaba et al. found that when a small amount of Na2HPO4, NaHCO; or Na2B4O7 was added to the Na2SO4 electrolyte, the specific capacitance of the manganese oxide electrode could be increased from 190F g-1 to 1.0A g-1 at the current density 200~230F·g-1, and the cycle performance is also significantly improved (>1000 cycles), which is due to the pH buffer provided by the additive to form a protective layer on the surface of manganese oxide, thereby inhibiting the dissolution of Mn.
Li et al. used FeSO4 and CuSO4 as sources of Fe2+ and Cu2+ to add sulfuric acid to supercapacitors as electrolyte redox additives. The specific capacity of the supercapacitor reaches 223mA·h·g1, which is much higher than that of the pure sulfuric acid electrolyte and the supercapacitor with only Cu2+ added sulfuric acid electrolyte. No redox peak was observed in the pure H2SO4 electrolyte, while the supercapacitor with the addition of Fe2+ and Cu2+ in sulfuric acid electrolyte exhibited better redox reversibility due to the synergistic effect of Fe2+ and Cu2+. Subsequently, Su et al. have used K3Fe(CN)6 and K4Fe(CN)6 as redox additives added in 1mol L-1KOH with Co-AI LDH (layered double hydroxide) as the electrode to study its pseudocapacitive performance. During the whole charge/discharge process, Co2+/Co3+ and Fe (CN)3-/6. Two independent redox reactions occur between /Fe(CN)4-/6. During the charging process, Co2+ loses an electron and is oxidized to Co3+, while Fe (CN)3-/6 gets an electron and is reduced to Fe. (CN)4-/6, in contrast, Fe (CN) is oxidized to Fe (CNCo3+ is reduced to Co2+.
Due to the presence of Fe(CN)3-/6/Fe(CN)4-/6 redox couple, the charge transfer resistance of KOH electrolyte decreased from 3.4%Ω to 2.96Ω. With CO-AI LDH (layered double hydroxide) as the electrode, the electrolyte is 1mol L-1 KOH, 1molL-1 KOH+0.1mol.L-1, K3Fe (CN) 6 and 1molL-1 KOH+0.1 When mol-L-1 K4Fe (CN) 6, the specific capacitance of the capacitors are: 226F·g-1, 712F·g-1 and 317F·g-1. However, the cycle stability of the above capacitors is poor. It was confirmed by XRD studies that no Fe(CN)3-/6/Fe(CN)4-/6 intercalation/deintercalation occurred inside Co-AILDH during the charge/discharge process. Generally speaking, in a layered structure, if an interlayer reaction occurs, corresponding peaks will appear in the X-ray diffraction pattern. Therefore, it is further confirmed that the Fe (CN)3-/6/Fe (CN)4-/6 Redox reactions only occur at the electrode/electrolyte interface or on the electrode surface, not inside the electrode.
Recently, it was reported that KI was added as a redox agent additive to 1mol L-1 H2SO4 electrolyte to improve the performance of porous activated carbon-based supercapacitors. The specific capacitance and energy density of supercapacitors after adding KI were 912F g-1 and 19.04W·h·kg-1, much higher than the values without KI (472F·g·1 and 9.5W·h·kg-1). Similarly, adding KBr to 1mol L-1H2SO4 can also increase the specific capacitance and energy density of the capacitor to 572F g-1 and 11.6W h kg-1.
In addition, VOSO4, as one of the most commonly used redox additives (VO2+/VO+/2,) for vanadium redox battery H2SO4 electrolyte, can improve the redox reversibility (VO2+/VO+/2) of the battery and the solubility of the electrolyte. Adding VOSO4 to 1mol L-1 H2SO4 electrolyte for carbon-based supercapacitors, a specific capacitance of 630.6F g-1 was obtained at 1mA cm-2, which was higher than that of 1mol L-1 H2SO4 alone (440.6F ·g-1) increased by 43%, and the energy density increased from 9.3W·h·kg-1 to 13.7W·h·kg-1 at 1mA·cm-2. In addition, after adding the additive, the internal resistance and equivalent series resistance of the capacitor decreased, and the cycle performance remained good (97.57%) after 4000 cycles.
Organic compounds, especially those with functional groups such as hydroquinone, quinone, and amine, are used as electrolyte mediators because of their ability to participate in electron transfer reactions. Roldyn et al. reported for the first time that hydroquinone was used as a redox active substance additive in 1mol L-1H2SO4 electrolyte, because hydroquinone is a good chemically active organic compound that can participate in the redox reaction of two-electron transfer. The redox characteristics of hydroquinone are mainly manifested in the ability to generate pseudocapacitance on the electrolyte electrode interface, thereby increasing the total capacitance of the supercapacitor. The maximum capacitance of this capacitor can reach 901F·g-1, at 2.65mA·cm- 2 The energy density is 31.3W·h·kg-1, the specific capacitance of this improved capacitor is nearly 3 times higher than that of 1mol-L-1HSO, electrolyte (about 320F·g-1), but the cycle stability is relatively low Poor, after 4000 cycles, the capacitance only retains 65% of the initial value. Similarly, Chen et al. reported the use of polystyrene rubber-graphene electrodes to add hydroquinone to 1mol L-1 H2SO4 electrolyte. The specific capacitance of the supercapacitor was 553F g-1, while 1mol L-1 H2SO4 The specific capacitance of the electrolyte supercapacitor is 280F g-1, and the capacitance is increased by 92%, which is due to the redox reaction of hydroquinone. In addition, the capacitance can still maintain 64% of the initial value after 50,000 cycles.
Subsequently, Jun et al. studied the performance of capacitors with different masses (0.010g, 0.025g, 0.050g, 0.075g and 0.100g) of p-phenylenediamine added to 1.78mol L-1 KOH solution, using MnO2 as the electrode material, and the results It is found that when the mass of p-phenylenediamine is 0.0050g, the maximum capacitance of the capacitor reaches 325.24F g-1 (1A g-1), which is nearly 6.25 times higher than that of pure KOH as the electrolyte, and the energy density increases from 1.29W h ·kg-1 increased to 10.12W·h·kg-1. The significant increase in its specific capacitance and energy density is attributed to the redox reaction between phenylenediamine/p-phenylenediamine. In addition, p-phenylenediamine changed the conductive mechanism of the electrolyte, and the electrolytic and charge transfer resistance of the supercapacitor dropped from 4.32Ω to 1.87Ω, and 4.68Ω to 2.87Ω. In addition, after 5000 cycles, the capacitor’s The specific capacity remained at 75% of the initial value. Similarly, they also reported the effect of adding the same redox agent additive in KOH solution on the performance of capacitors using activated carbon as an electrode. After adding terephthalate to KOH solution, the supercapacitor has higher specific capacitance and energy density: 605.225F·g-1 and 19.862W·h·kg-1, while the specific capacitance and energy density of pure KOH are: 144.037F·g-1 and 4.458W·h·kg-1, after 4000 cycles, the capacitance of the supercapacitor still retains 94.53% of the initial value. In a supercapacitor with single-walled carbon nanotubes as electrodes, the specific capacitance and energy density values of the same electrolyte (terephthalene + KOH) are 162.66F·g-1 and 4.23W·h·kg-1, after the improvement The performance of the supercapacitor is 4 times that of using pure KOH as electrolyte.
Besides, m-phenylenediamine, another novel redox additive, was also used in KOH electrolyte to enhance the performance of supercapacitors through its electrochemical redox process. When m-phenylenediamine was added to the KOH electrolyte, the equivalent series resistance of the supercapacitor decreased from 2.60Ω·cm2 to 1.98Ω·cm2, and the capacitance increased from 36.43F·g-1 to 78.01F·g-1 after 1000 cycles It still has good electrochemical performance after cycling.
Sodium indigo disulfonate is also used as a redox additive because of its two
Quinone and amine functional groups mainly involved in redox reactions. The mechanism of redox reaction in 1mol L-1 H2SO4 electrolyte is as follows: Two electron transfer reactions occur at the quinine site, and sodium indigo disulfonate is oxidized and reduced to white sodium indigo disulfonate, and then passes through the amine site The two-electrode transfer reaction on the point further participates in the redox reaction, and finally converts sodium indigo disulfonate into sodium dehydroindigo disulfonate. The redox peak of this process can be observed in its cyclic voltammogram. When sodium indigo disulfonate is added to 1mol L-1H2SO4 electrolyte, the specific capacitance of the capacitor increases from 17F·g-1 to 50F·g-1, and the energy density increases from 0.6W·h·kg- to 1.7W· h kg-1 Moreover, the capacitance value retained 30% of the initial value after 10,000 cycles. Due to the reversible electrochemical behavior of methylene blue, it is used as a redox mediator and is known as an organic dye.
Adding methylene blue to 1mol L-1 H2SO4 electrolyte can increase the specific capacitance of activated carbon supercapacitor from 5F g-1 to 23F g-1, because in the redox process, 3H+ is generated or adsorbed through 2e-gain and loss, and the cycle is 6000 After one cycle, the capacitance value remained at 88% of the initial value. Adding sodium lignosulfonate to 1mol L-1 H2SO4 electrolyte can also increase the specific capacitance of the capacitor. When the electrolyte is pure sulfuric acid, the specific heat capacity of the capacitor is 145F g-1: after adding sodium lignosulfonate, the specific heat capacity is 178F g -1.
Dissolve humic acid into 6mo L-1 KOH solution to prepare electrolytes with different concentrations (mass fractions: 2%, 5%, and 10%). Humic acid and its potassium salt dissolve in water to form dark brown solution, the solubility is small, and when the content of humic acid is close to 10%, the solution shows a state close to saturation, and even after a long time of stirring, a small amount of viscous aggregates can still be retained at 25°C, using stainless steel electrodes and PTFE gasket (cell constant 0.588cm-1) to determine its conductivity (see Table 5-4). As the content of humic acid increases, the conductivity of the solution decreases significantly. When the concentration of humic acid reaches the highest value, the resistance of the capacitor is about half of that of pure 6mol L-1 KOH (the conductivity of 6mol L-1 KOH is 0.627S cm-1). The addition of humic acid resulted in a slight increase in viscosity, which resulted in a decrease in ion mobility, and with increasing humic acid content, the conductivity of the solution dropped to 0.347S· cm-1.
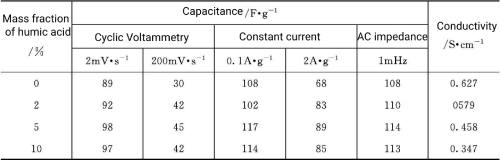
Table 5-4 Performance of supercapacitors with different mass fractions of humic acid added as an additive to 6molL-1 KOH electrolyte
Compared with the pure electrolyte, the supercapacitor with the electrolyte added with humic acid additive not only showed higher capacitance performance during the whole cycle, but also showed a smaller drop in capacitance value after 5000 cycles. Table 5-4 shows the performance parameters of capacitors containing different humic acid additives. It is not difficult to see that the performance of capacitors is significantly improved after adding humic acid.
When the mass fraction of humic acid is 5%, it has the greatest impact on the properties of the capacitor. When the mass fraction of humic acid exceeds 5%, the conductivity decreases, the ion mobility decreases, and the capacitance decreases slightly.
Due to the presence of chemically active oxygen groups, including carboxyl, phenolic, and alcoholic hydroxyl groups, humic acid species will interact with the surface of carbon with similar chemical and physical properties to provide conditions for redox reactions. The interaction analysis based on hydrogen bonds will lead to a more complicated discussion of the kinetic process, because humic acid can also form stable covalent bonds with the surface medium of carbon, resulting in the increase of oxygen functional groups in the electrolyte and the hydrophilicity of the carbon interface. However, the modification method of this electric double layer structure remains to be understood. Due to the complex chemical properties of humic acid, the process involves many phenomena and mechanisms, and the related system research will become the subject of in-depth research in the future.
4.2 Redox active liquid electrolytes
Refers to electrolytes that can directly involve charge transfer reactions or redox reactions without any additional redox additives. They can act as both electrolytes and redox mediators. Figure 5-2 is a simple redox-active electrolyte charge storage mechanism. In this case, ions are adsorbed from the electrolyte to the electrode surface, and these adsorbed ions directly participate in redox reactions at the electrode/electrolyte interface, and these electrochemical redox reactions are reversible. For example, K4Fe(CN)6 can serve as an electrolyte for charge storage and also as a redox mediator, since Fe(CN)4-/6 participates in the redox reaction to transform into Fe(CN)3-/6.
The advantage of this type of electrolytes is that they are economical and safe, and these electrolytes can be directly used as electrolytes and redox active materials without adding any additional redox additives.
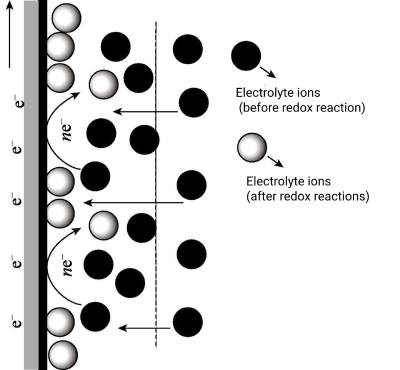
Figure 5-2 Electrochemical redox processes in redox-active electrolytes
Lota et al. used KI as a redox electrolyte in a supercapacitor. At a scan rate of 1mV s-1, the maximum capacitance obtained by KI as the electrolyte of a supercapacitor was 261F g-1, which was larger than that of sulfuric acid ( 160F·g-1). Subsequently, the research group investigated the performance of various metal iodide solutions as electrolytes, such as Lil, Nal, Rbl, KI, and Csl, among which KI and Csl showed the largest specific capacitance (about 234F g-1), and Nal, The specific capacitances of Rbl and Lil are 203F·g-1, 220F·g-1 and 178F·g-1, respectively. In addition, K4Fe(CN)6 can also be used as a redox-active electrolyte for electrochemical double-layer capacitors. During the entire charging/discharging process, two reactions exist simultaneously: the adsorption/desorption of Fe (CN)4-/6 and the redox reaction of Fe (CN)4-/6/Fe (CN)3-/6, These two reactions take place on the positive electrode, and at a voltage of 1.2V, a maximum specific capacity of 272F·g-1 is obtained. In most cases, I- and Fe (CN)4-/6 will undergo redox reactions on the positive electrode, so the maximum specific capacitance may come from the positive electrode rather than the negative electrode.
Frackowiak et al. introduced a new approach to balance two equal anode and cathode electrodes by using two different redox-active electrolytes (KI and VOSO4) on the same supercapacitor (one for the anode and the other for the anode). capacitance to improve the performance of supercapacitors. These two electrolytes are separated by a separator. Typically, for this type of energy storage system, a separator is used to circumvent the mixing of electrolytes and complete the current circuit by transporting non-reactive species of anions or cations. The membrane used in this study was a proton (cation) exchange membrane, and this new system showed good specific capacitance (700F g-1) and energy density (20W h kg-1) at 0.5A g-1. 1) .
In recent years, supercapacitors have been widely used, but their low energy density compared with batteries is a long-standing problem. In the past few decades, many efforts have been made to solve this problem, mainly to find new electrode materials and more suitable electrolytes. Redox additives or redox active ions can change the ionic conductivity of the electrolyte and the specific capacitance of the capacitor through the redox reaction that occurs at the electrode/electrolyte interface. The specific capacitance and energy density of these capacitors are almost close to the values of hybrid capacitors, and their energy density values are also comparable to some batteries. Compared with the research and development of electrode materials, the modification of electrolyte is simpler and economical.
Although many methods have been proposed in the literature to improve the performance of supercapacitors through the use of redox additives/active electrolytes, no clear studies have shown the optimal conditions for the electrolyte. In future work, fundamental theoretical and commercial More research is needed for its application. There will be many opportunities for research in this area, but also note the following:
① There are many kinds of electrode materials, but only a few materials are suitable for the research of redox active electrolytes.
② Redox additives are mainly used in supercapacitors aqueous electrolyte and have not been tested in organic electrolytes.
③ These types of electrolytes have only been studied in symmetric supercapacitors, and have not yet begun to be used in asymmetric supercapacitors.
④ There are many redox-active materials or compounds available, but they have not been used as redox additives for electrolytes in supercapacitors.
If the above problems can be solved in the future, it will further accelerate the application of redox additives/active electrolytes in supercapacitors. Therefore, we hope that this field of research will develop rapidly in the near future, leading to improved performance of supercapacitors.