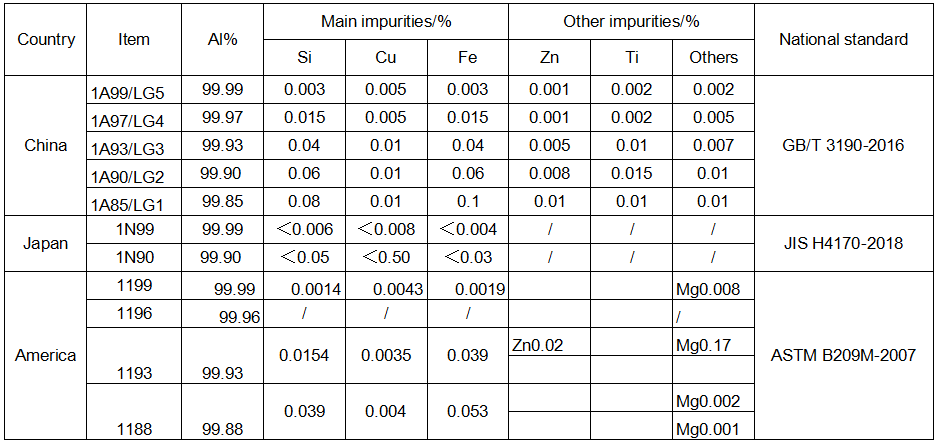
🔊The purity of electrolytic capacitor electrode aluminum foil listed in the standards of China, Japan, the United States and other countries is as follows.
🌟In addition to purity, high-purity aluminum foil with the same aluminum purity will also cause differences in capacitor performance due to different trace impurities. At the same time, it will also have a great impact on the corrosion of the aluminum foil and the energizing performance of the oxide film.
🌟The impurity in the aluminum foil of the electrolytic capacitor electrode focuses on iron, silicon, and copper.
⚫️The impurity copper element and aluminum element also belong to the face-centered cubic lattice configuration. Because of the similar compatibility principle, the degree of solid dissolution of copper as an impurity in aluminum is quite high.
At 548°C, the solubility of copper in aluminum can reach 6.65%; when the temperature drops to 302°C, the solubility of copper is still 0.45%, so there are almost no isolated copper grains in high-purity aluminum.
⚫️The solubility of impurity silicon in aluminum solid solution is 1.65% at the eutectic temperature of Al-Si (11.7% silicon) at 577°C. Although the solubility of silicon drops sharply as the temperature drops, silicon and aluminum can form alloys.
⚫️The amount of impurity iron in the solid solution of aluminum is extremely low, but if there is an appropriate amount of impurity silicon at the same time, the impurity iron that cannot be dissolved can combine with silicon and aluminum to form a ternary compound, which exists between the grain boundaries and is The grain boundary corrosion process is ruled out. When the silicon content is greater than iron, β-FeSiAl3 (or Fe2Si2Al9) phase is formed, and when the iron content is greater than silicon, α-Fe2SiAl8 (or Fe3Si2Al12) is formed.
Excessive iron impurities can also form dispersed iron aluminide binary compounds with aluminum. Typical representatives such as Fe3Al, FeAl, etc., appear in granular form, which adversely affects the performance of the energized aluminum oxide film.
If the particle size of the aluminum-iron intermetallic compound is larger than the thickness of the dielectric film, it is equivalent to setting up a conductive bridge that is easy to conduct leakage current in the highly insulating oxide layer, so the impact of impurity iron is the most serious.
🌟On the contrary, there is a trace amount of impurity magnesium in aluminum, which is easy to form the nucleation center MgAl2O4, which belongs to the spinel structure, which can promote the accelerated transformation of aluminum oxide to the crystalline state. The more nucleation centers in the aluminum material, the greater the number of tiny crystals. Obviously, this is beneficial to the manufacture of low-voltage aluminum foil, so sometimes there is the behavior of active doping of Mg.
The trace impurities in the aluminum foil and aluminum form a ZrAl3 compound, which can prevent the recrystallization process and help refine the recrystallized grains. Similarly, rare earth elements can also play a role in refining crystal grains.